Abstract
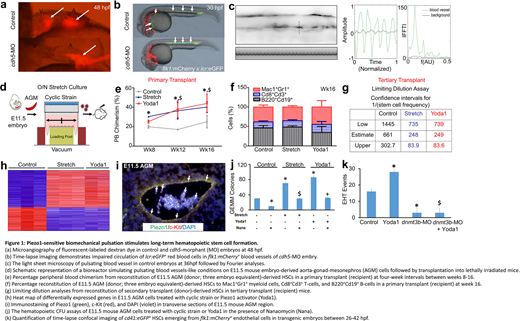
The birth and development of hemat\opoietic stem cells (HSCs) remain a mystery. During fetal development, a subset of endothelial cells transitions to become HSCs in the aorta-gonad-mesonephros (AGM) region. Blood flow-mediated shear stress and activation of nitric oxide synthase (NOS) were demonstrated to stimulate the endothelial-to-HSC transition in the AGM. However, we showed that malbec (mlbbw306), a zebrafish mutant for cadherin 5, produces HSCs despite circulation arrest and the inhibition of NOS, suggesting that other biomechanical forces, mechanosensation pathways, or epigenetic mechanisms might regulate HSC formation and could have utility in developing HSCs. Using zebrafish, murine, and human models, we show that Piezo1-sensitive biomechanical stretching of hemogenic endothelial cells enhances Dnmt3b expression for long-term (LT)-HSC formation.
Our microangiography and time-lapse confocal imaging established that cdh5-MO embryos have a heartbeat and pulsation in blood vessels despite the absence of blood flow. We also employed light sheet and time-lapse confocal microscopy followed by Fourier transform analyses to establish that although pulsation is independent of blood flow in the AGM, it is concurrent with the endothelial-to-hematopoietic transition. To establish the functional link between pulsation and HSC formation, we developed a bioreactor simulating the pulsating blood vessel conditions. We found that the biomechanical stretching of hemogenic endothelial cells or the pharmacological activation of Piezo1 yields three times higher amounts of LT-HSC formation; which reconstitute to normal multi-lineage adult blood even upon serial transplantation. Our gene-silencing, time-lapse imaging, explant culture, and computational analyses further demonstrated that biomechanical stretching activates Piezo1; which enhances epigenetic regulator Dnmt3b expression to stimulate the endothelial-to-HSC transition.
Our results demonstrate how pulsation-mediated biomechanical forces stimulate cell-fate transitions and stem cell formation by activating mechanosensitive channels as well as epigenetic machinery. We present a model that addresses major challenges in HSC transplantation and cellular therapies for treating blood and bone marrow diseases. In addition, we report a scalable bioreactor with potential widespread use and a pharmacological target to develop and expand LT-HSCs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal